Abstract
Introduction. Philadelphia negative (Ph-neg) Myeloproliferative Neoplasms (MPNs) differ in clinical phenotype and outcomes despite harboring identical driver mutations. The biology behind phenotypic heterogeneity has been attributed to mutational burden, co-occurring mutations and mutation order, but remains uncertain. It is known that MPN mutations in JAK2, CALR, and MPL result in augmentation of cytokine signaling at different stages of hematopoietic differentiation towards myeloid lineages. Consistent with this, the JAK2V617F driver mutation is enriched in certain myeloid lineages compared to the hematopoietic stem and progenitor cell (HSPC) compartment (Anand et al. Blood 2011). We therefore hypothesized that the lineage-specific patterns of clonal enrichment that arise during hematopoietic differentiation from stem cells to mature progeny would account for phenotypic heterogeneity in MPNs.
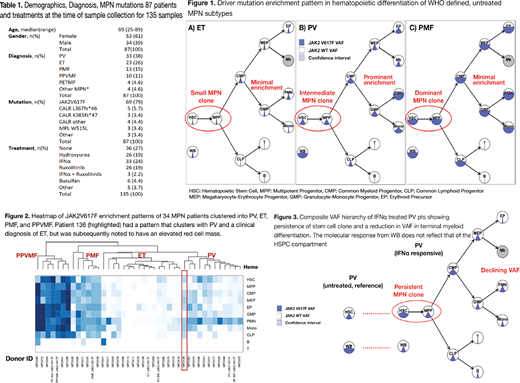
Methods. Peripheral blood and/or bone marrow specimens were prospectively collected from 87 MPN patients (pts) providing informed consent between July 2017 and July 2018. Data on age, gender, diagnosis, date of diagnosis, symptoms, spleen size, blood counts, bone marrow findings, mutation profile, and treatment was collected for all pts. Each specimen was deconvoluted into 11 well-defined and strictly validated hematopoietic populations using a combination of density gradient separation (Ficoll), immunomagnetic selection (CD34) and fluorescence-activated cell sorting (FACS). Final purification of specimens was performed by multiparameter FACS to isolate CD15+/CD16+ polymorphonucleated cells (PMNs), CD14+/CD11b+ monocytes, CD3+ T cells, CD19+ B cells, CD71+/CD36+ erythroblasts and 6 well-defined HSPC populations (Manz et al. PNAS. 2002, Majeti et al. Cell Stem Cell 2007). Functional and morphologic characteristics were validated for all populations. DNA was extracted from the sorted populations and the variant allelic frequency (VAF) of the driver mutation in JAK2, CALR, or MPL was quantified by droplet digital PCR. Individual patterns of mutation enrichment were represented by the VAF plotted on a hematopoietic hierarchy (Fig 1). A composite VAF hierarchy was established for each of the WHO defined MPN subtypes of Polycythemia Vera (PV), Essential Thrombocythemia (ET), and Primary Myelofibrosis (PMF) prior to treatment, during course of treatment, and in secondary myelofibrosis. Clustering of individual patterns in relation to composite trees was performed using principal component analysis (PCA), which allowed clinical validation of clustering patterns.
Results. A total of 135 samples were collected from 87 pts. Demographic and clinical features of the cohort are shown in Table 1. The composite pattern of MPN mutation enrichment differed in PV, ET and PMF (Fig 1). Pts with untreated PV or ET harbored a small proportion of mutated stem cells. The VAF in PV patients implied clonal dominance in mature myeloid progeny while there was minimal enrichment of the driver mutation in pts with JAK2 ET. In comparison, pts with PMF had a significantly higher VAF in the HSC compartment, but had minimal enrichment of the driver mutation during myeloid differentiation. Using PCA, individual patterns from a sample of 34 JAK2V617F MPN pts were clustered in reference to the pre-established composite patterns (heatmap in Fig. 2). This was done in order to validate consistency of patterns with clinical diagnoses (see example in Fig 2).
Finally, we found that interferon treated PV pts had a unique decline in VAF with myeloid differentiation (Fig 3) but persistence of the mutant allele within the stem cell compartment: a finding that potentially explains the persistence of bone marrow abnormalities in pts achieving molecular response determined by whole blood (WB) VAF.
Conclusion. The pattern of clonal enrichment of an MPN stem cell during hematopoiesis is unique to individual pts and varies significantly among the 3 major WHO subtypes of PV, ET, and PMF. This pattern also differs in relation to the disease stage, and is informative of treatment effects. WB VAF does not predict the proportion of immature HSPC harboring driver mutation. For these reasons, evaluating the clonal heterogeneity of MPN driver mutations, particularly those in HSPCs, may provide a useful surrogate measure to qualify response to novel agents in pre-clinical and clinical studies in MPNs. This is currently being tested.
Ritchie:NS Pharma: Research Funding; Incyte: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Research Funding; ARIAD Pharmaceuticals: Speakers Bureau; Astellas Pharma: Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Celgene: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal